Disinfection
UV-C Disinfection
When bacteria, viruses and protozoa are exposed to the germicidal wavelengths of UV-C light, they are rendered incapable of reproducing and infecting. Microorganisms are inactivated by UV-C light as a result of damage to nucleic acids. The high energy associated with short wavelength UV-C energy, primarily at 254 nm, is absorbed by cellular RNA and DNA. This absorption of UV-C energy forms new bonds between adjacent nucleotides, creating double bonds or dimers. Dimerization of adjacent molecules, particularly thymine, is the most common photochemical damage. Formation of numerous thymine dimers in the DNA of bacteria and viruses prevents replication and inability to infect.
- UV-C is a chemical-free process
- UV-C requires no transportation, storage or handling of toxic or corrosive chemicals: a safety benefit for plant operators and the surrounding community
- UV-C treatment creates no carcinogenic disinfection by-products that could adversely affect quality
- UV-C is highly effective at inactivating a broad range of microorganisms including chlorine-resistant pathogens like Cryptosporidium and Giardia
- UV-C can be used to break down toxic chemical contaminants while simultaneously disinfecting.
- Annual lamp replacement and electrical consumption comprise the operating costs of UV-C disinfection
- UV-C eliminates or reduces the immediate safety threat of chlorine gas without creating new long term costs associated with chemicals, transportation and delivery
- • Costs for leak response, administration, risk management and emergency planning and operator training are minimized and/or eliminated with UV-C
Ozone disinfection
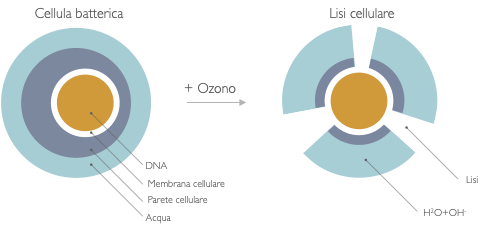
Microorganisms cause issues in various places, in a clinical setting bacteria can cause dangerous outbreaks. Ozone can be used as a chemical disinfectant to kill bacteria and viruses with low ozone concentrations. The contact time is altered depending on the desired deactivation grade. Non-touch technologies include the usage of UV-C lamps and chemicals dispersed as an aerosol or gas which deactivates microorganisms. Compared to other treatment methods for air disinfection, ozone can efficiently disinfect large air volumes, neutralizing micro-organisms, including viruses. This makes it ideal for use in medical applications, for example in hospitals or doctors waiting rooms. An important factor that enables savings is the time the cleaning agent can actively deactivate bacteria.
- UV-C cleaning systems often have a very short time window to irradiate the air and therefore needs to add a lot of energy to ensure sufficient deactivation in this short time, wiping with a cleaning solution is limited by the time it takes for the surface to dry while ozone will continue to attack bacteria until it naturally decomposes or is removed. This enables ozone solutions to increase energy saving significantly.
- Ozone is produced on-site from the oxygen in ambient air, an abundant free-of-cost raw material. Nothing to purchase, transport and store.
- No handling and refills needed
- No waste
- No residues
- Our combined ozone systems also save time and money.
- The operation is automatic with minimal maintenance and very affordable operating cost
|
Chlorine Disinfection |
UV-C Disinfection |
|
|
Disinfection with products |
Yes |
No |
|
Chemical Residues |
Yes |
No |
|
Corrosive |
Yes |
No |
|
Community security risks |
Yes |
No |
|
Efficacy on Cryptosporidium and Giardia |
No |
Yes |
